Introduction
BAY 94-9027 (damoctocog alfa pegol) is an extended-half-life PEGylated recombinant factor VIII approved for use in patients with hemophilia A aged ≥12 years (USPI; https://www.fda.gov/). Due to age-related pharmacokinetic and other differences to adult patients, there is a need to assess FVIII replacement products in pediatric populations (Shah et al, Haemophilia 2018, 24(5):733-40). In the PROTECT VIII Kids main study (NCT01775618), BAY 94-9027 was efficacious for the prevention and treatment of bleeds in previously treated patients (PTPs) aged <12 years with severe hemophilia A. We report long-term efficacy and safety data from the PROTECT VIII Kids extension in patients aged <6 and 6 to <12 years at study entry.
Methods
In PROTECT VIII Kids, male PTPs aged <12 years with severe hemophilia A were enrolled in two age cohorts: <6 and 6 to <12 years. Patients received BAY 94-9027 prophylaxis twice weekly (25-60 IU/kg), every 5 days (E5D, 45-60 IU/kg), or every 7 days (E7D, 60 IU/kg) at the investigator's discretion. Patients completing ≥50 exposure days and ≥6 months in the main study, or a 12-week safety expansion study that enrolled additional PTPs aged <6 years, could continue in the optional extension phase. Patients recorded bleeds and BAY 94-9027 usage in an electronic diary and were followed for safety assessments every 6 months. Analyses were based on age group at enrollment (<6 or 6 to <12 years). The study complied with the principles found in the Declaration of Helsinki.
Results
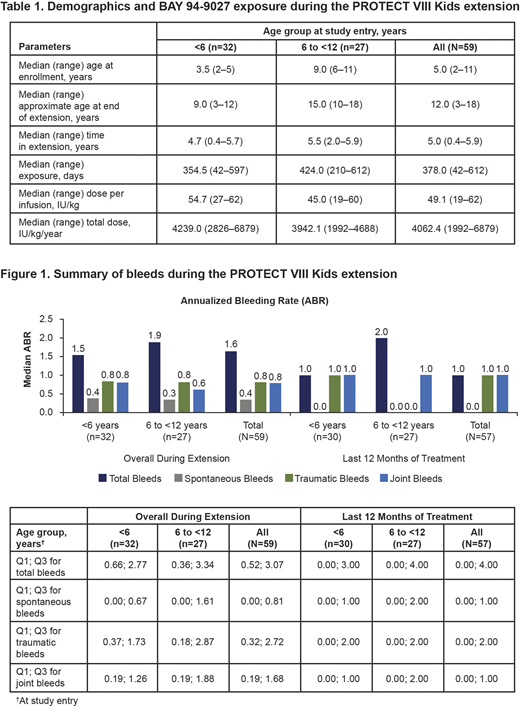
Of the 73 PTPs enrolled in the main or expansion study, 59 continued in the extension phase (n=32 aged <6 years; n=27 aged 6 to <12 years). Median age at enrollment was 5.0 years and median age at the end of the extension was 12.0 years. Median time in the extension study was 4.7 years in patients aged <6 years and 5.5 years in those aged 6 to <12 years; median exposure was 354.5 and 424.0 days, respectively (Table 1). Fifty-two patients completed ≥3 years of treatment and 39 patients completed ≥5 years. At the end of the extension, 29 patients were treated twice weekly, 20 patients were treated E5D and 10 patients were treated E7D. The median number of infusions per year was 78.
Median annualized bleeding rate (ABR) for total bleeds was 1.54 for all patients aged <6 years and 1.89 for those aged 6 to <12 years. Total ABR improved compared to the main study and was maintained during the extension. In the last 12 months of treatment, median spontaneous ABR was 0.0 in both age groups, however, trauma ABR was higher in patients aged <6 years (Figure 1). In the last 12 months, 19 patients (33.3%) had zero bleeds and 27 patients (47.4%) had zero joint bleeds. Overall, joint bleeds comprised 49.2% and 46.5% of total bleeds in younger and older patients, respectively, and predominantly affected ankle, knee and elbow joints. The majority of bleeds were mild (<6 years, 52.9%; 6 to <12 years, 30.8%) or moderate (<6 years, 39.3%; 6 to <12 years, aged <6; 55.5%) in severity.
During the extension, 4 patients (6.8%) experienced study-drug-related adverse events (AEs): 1 patient (<6 years) had suspected FVIII inhibitors; 1 patient (<6 years) had severe muscle spasms; 1 patient (6 to <12 years) had suspected FVIII inhibitors; and 1 patient (6 to <12 years) had both suspected FVIII inhibitors and mild arthralgia. Two patients in the older group reported study-drug-related serious AEs (SAEs) of suspected FVIII inhibitors, neither of which was confirmed. Two patients aged <6 years discontinued, one due to an AE and one due to a non-study-drug-related SAE. No safety signals related to hypersensitivity or loss-of-efficacy were detected. A total of 11 patients (18.6%) had detectable PEG in plasma during the extension (n=6 aged <6 years; n=5 aged 6 to <12 years). PEG was detected at only a single time point in 6 patients and at repeated time points in 5 patients; in all cases, PEG levels were just above the detection limit (0.1 mg/L) and did not change over time, indicating steady-state was reached. Levels of renal biomarkers (Albumin, Beta-2 Microglobulin, Cystatin C, Kidney Injury Molecule-1, Lipocalin-2, proteinuria) were consistent over time, demonstrating normal renal function.
Conclusion
In both younger and older pediatric patients with severe hemophilia A, long-term treatment (median 5.8 years) with BAY 94-9027 prophylaxis was efficacious and well-tolerated.
Financial support: Study funded by Bayer.
Ahuja:Genentech: Consultancy; Sanofi-Genzyme: Consultancy; XaTec Inc.: Consultancy; Sanofi-Genzyme: Honoraria; Genentech: Honoraria; XaTec Inc.: Research Funding; XaTec Inc.: Divested equity in a private or publicly-traded company in the past 24 months. Fischer:Bayer, Baxter/Shire, SOBI/Biogen, CSL Behring, Octapharma, Pfizer, NovoNordisk: Research Funding; Bayer, Biogen, Pfizer, Baxter/Shire, and Novo Nordisk: Research Funding; Bayer, Baxter, Biogen, CSL Behring, Freeline, Novo Nordisk, Pfizer, Roche, and Sobi: Consultancy. Biss:Bayer, Boehringer Ingelheim: Honoraria. Maas Enriquez:Bayer: Current Employment. Mancuso:Bayer, CSL Behring, Novo Nordisk, Roche, Sobi, Octapharma, Kedrion, Pfizer, Shire/Takeda, Biomarin, Grifols: Speakers Bureau; Bayer, CSL Behring, Novo Nordisk, Roche, Sobi, Octapharma, Kedrion, Shire/Takeda, Biomarin, Grifols, Catalyst: Honoraria; Bayer, CSL Behring, Novo Nordisk, Roche, Sobi, Octapharma, Kedrion, Pfizer, Shire/Takeda, PedNet Foundation: Consultancy. Wang:Bayer: Current Employment. Kenet:Bayer, Pfizer, Takeda, BioMarin, Novo Nordisk: Speakers Bureau; PI Healthcare, CSL Behring: Honoraria; Bayer, Pfizer, Roche, Alnylam (Sanofi), Shire: Research Funding; Bayer, Pfizer, BioMarin, Takeda, Roche, Novo Nordisk, Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
BAY 94-9027 (damoctocog alfa pegol), current indication for patients with hemophilia A aged >12 years. We report efficacy and safety data in patients aged <12 years.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal